Selecting the right Contract Development and Manufacturing Organization (CDMO) for your oral liquid syrup is one of the most critical decisions you will make in your product’s lifecycle. A great partner accelerates your path to market, while the wrong one can lead to costly delays, quality issues, and regulatory hurdles. This guide provides the essential criteria and a step-by-step process for selecting a reliable CDMO for your pharmaceutical syrup project, ensuring you make a confident and informed choice.
Why Partner with a Specialized Pharmaceutical Syrup CDMO?
While many manufacturing partners exist, a CDMO specializing in oral liquids offers distinct advantages. It’s crucial to first understand the difference: a Contract Manufacturing Organization (CMO) typically handles production based on your provided formula, whereas a CDMO provides comprehensive development services alongside manufacturing. For complex formulations like syrups, this integrated expertise is invaluable.
Specialized syrup CDMOs are equipped to handle the unique challenges inherent to liquid dosage forms. This includes advanced expertise in:
- Taste-masking, viscosity, and stability: Syrups require a delicate balance of active pharmaceutical ingredients (APIs), excipients, and flavoring agents to ensure efficacy, a palatable taste, and a stable shelf life.
- Complex formulations: Developing effective and safe syrups for specific patient populations, such as pediatric or geriatric patients, requires specialized knowledge of dosing, safety, and palatability.
- Microbial control: Aqueous-based syrups are susceptible to microbial growth, demanding robust preservative systems and stringent environmental controls during manufacturing to ensure product safety.
Key Services to Expect from a Syrup CDMO
A true end-to-end syrup CDMO partner should offer a comprehensive suite of services that covers the entire product lifecycle. Look for a partner capable of providing:
- Pre-formulation and formulation development: Initial studies to characterize the API and develop a stable, effective, and marketable syrup formulation.
- Analytical method development and validation: Creating and validating precise testing methods to ensure product quality, potency, and purity at every stage.
- Clinical trial manufacturing and scale-up: Producing batches for clinical studies and seamlessly scaling the process from the lab to commercial-sized equipment.
- Commercial manufacturing and packaging: Full-scale, GMP-compliant manufacturing, bottling, labeling, and packaging for market supply.
- Regulatory submission support: Expert assistance in preparing the critical Chemistry, Manufacturing, and Controls (CMC) section of your regulatory filings.
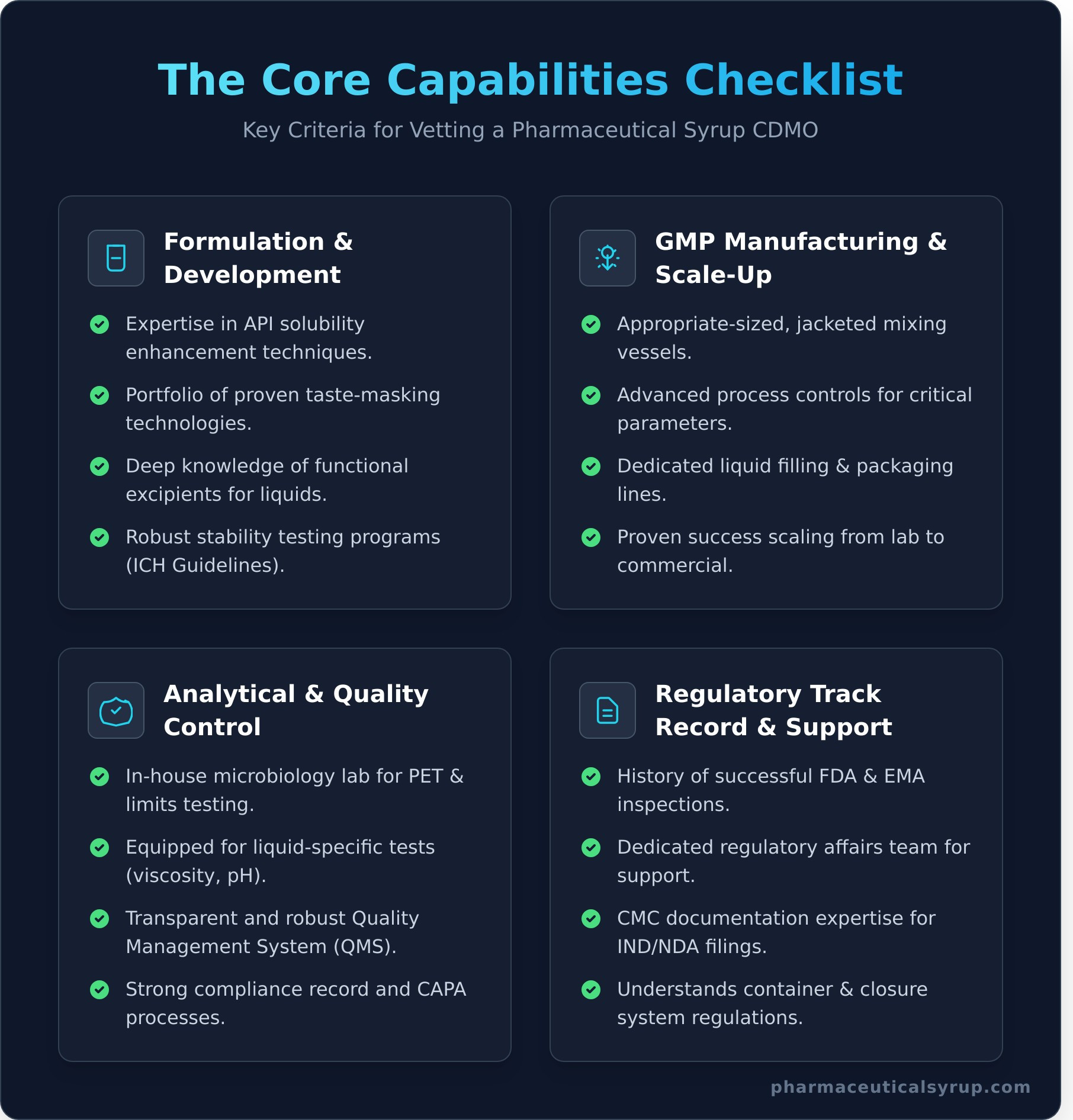
The Core Capabilities Checklist for Vetting a Syrup CDMO
Use the following checklist as your framework for evaluating and comparing potential partners. Don’t just take their word for it; seek tangible evidence of their expertise and capabilities in these critical areas.
Formulation and Development Expertise
A CDMO’s scientific depth is the foundation of a successful project. Verify their experience with API solubility enhancement techniques, as many APIs are challenging to dissolve in a syrup base. They should have a portfolio of proven taste-masking technologies to ensure patient compliance, especially for bitter APIs. Inquire about their knowledge of functional excipients for oral liquids and their approach to conducting robust stability testing programs according to ICH guidelines.
GMP Manufacturing and Scale-Up Capabilities
The facility and equipment are paramount. Ensure the CDMO has appropriate-sized, jacketed mixing vessels to accommodate your projected batch needs and maintain precise temperature control. Advanced process controls for monitoring viscosity and other critical parameters are non-negotiable. Look for dedicated liquid filling and packaging lines to prevent cross-contamination. Most importantly, ask for specific examples of how they have successfully scaled a product from lab development to commercial production. A well-equipped facility is the cornerstone of quality manufacturing; see our state-of-the-art liquid manufacturing facility.
Analytical and Quality Control Systems
A strong quality unit is your best insurance policy. The CDMO must have an in-house microbiology lab capable of performing microbial limits testing and Preservative Efficacy Testing (PET). Their analytical lab should be equipped for key liquid-specific tests, including viscosity, pH, and particle size analysis. Dig deep into their Quality Management System (QMS) and ask about their compliance record, deviation management, and CAPA processes.
Regulatory Track Record and Support
A CDMO is more than a manufacturer; they are your regulatory partner. Investigate their history of successful inspections by major regulatory bodies like the FDA and EMA. They should have a dedicated regulatory affairs team with demonstrable experience in preparing the CMC documentation for IND and NDA filings. Furthermore, confirm their understanding of the complex regulatory requirements for container and closure systems, which are critical for ensuring the stability and safety of liquid products.
Your Step-by-Step Guide to the CDMO Selection Process
Following a structured selection process will help you minimize risk and ensure you find a partner that aligns perfectly with your technical, quality, and business needs.
Step 1: Create a Detailed Request for Proposal (RFP)
A thorough RFP is the first step to getting meaningful and comparable quotes. Be sure to include your API details and a clear target product profile. Define the specific services you require, from development through commercial supply. It is also critical to specify your target markets (e.g., US, EU) to address relevant regulatory requirements and provide estimated annual volume forecasts to help the CDMO assess project feasibility.
Step 2: Conduct Due Diligence and Audits
Once you have shortlisted potential partners, the deep dive begins. Review the CDMO’s quality history and any publicly available regulatory records, such as FDA 483s or warning letters. Ask for non-confidential case studies or examples of projects similar to yours. Finally, schedule a comprehensive quality audit, either virtually or in-person, to see their operations, meet the team, and verify that their systems and facility meet your standards.
Step 3: Evaluate the Proposal and Quality Agreement
When you receive proposals, look beyond the bottom line. Compare project timelines, cost structures, and what is included in the price. The draft Quality Agreement is a critical document; review it carefully with your quality team to ensure it clearly defines roles, responsibilities, communication plans, and quality metrics. A transparent and collaborative approach to this document is a strong indicator of a good partner. When you’re ready to take the next step, request a proposal for your syrup project today.
Frequently Asked Questions
What is the difference between a CMO and a CDMO for syrups?
A CMO (Contract Manufacturing Organization) primarily focuses on manufacturing a product based on a formula you provide. A CDMO (Contract Development and Manufacturing Organization) offers a broader partnership, providing services that span from early-stage formulation development and analytical testing all the way through commercial manufacturing and regulatory support.
How is intellectual property (IP) handled when working with a CDMO?
IP ownership should be clearly defined in your development and supply agreements. Typically, the client retains ownership of their molecule and the final product formulation. The CDMO may own pre-existing background IP related to their specific processes, but this should be licensed to you for the purposes of your product.
What are the first steps in the technology transfer process for a syrup?
The first steps involve assembling a joint project team and exchanging detailed technical information. This includes sharing all data on the API, existing formulation work, analytical methods, and stability data. The CDMO will then typically perform lab-scale feasibility and verification work before moving to larger-scale batches.
How long does it typically take to go from formulation to commercial batch?
The timeline can vary significantly based on project complexity, but a typical range might be 18-24 months. This includes formulation development, analytical method validation, stability studies (which can take 6 months or more), process scale-up, and manufacturing of validation batches required for regulatory submission.
What are the most common red flags to watch for when selecting a CDMO?
Key red flags include poor communication or lack of transparency, a history of significant regulatory compliance issues, high staff turnover (especially in quality or technical roles), and an unwillingness to be flexible or collaborative in structuring the Quality Agreement.
Choosing the right CDMO is a strategic decision that profoundly impacts your product’s success. By focusing on specialized expertise in oral liquids, verifying core capabilities, and following a structured evaluation process, you can build a lasting partnership that navigates challenges and successfully brings your pharmaceutical syrup to market. With over 20 years of specialized oral liquid experience in FDA and EMA-inspected GMP facilities, we have successfully developed more than 50 unique syrup formulations. Partner with us to develop and manufacture your next oral liquid product.



